Year in Review: 2016
A Summary of Autism Discoveries in 2016 and What It Means for Families
By Alycia Halladay, PhD, Chief Science Officer of the Autism Science Foundation and the Scientific Advisory Board of the Autism Science Foundation

To listen to our year-end research summary podcast, click here.
For decades, the autism community has known that autism affects the entire family. Biological parents have been included in autism studies to examine where genetic mutations come from, but always with an eye for understanding the affected individual. This year in research saw a much bigger focus on family members of those with autism, particularly siblings. The goal of these studies is to understand the genetic and biological nature of autism so that help can be provided not just to those with a diagnosis, but to family members as well.
Many studies focused on what is known as the “broader autism phenotype,” previously explored in biological parents. The “broader autism phenotype” refers to some behavioral features of autism, including those in emotion, language, and social skills that do not meet the level of a diagnosis of autism spectrum disorder. Rather, they have been termed anything from “intermediate” autism to “a hint of autism.” Joe Piven and James Harris hypothesized this year that Bruno Bettleheim may have tragically misinterpreted these features, in the absence of a true understanding of autism, as “refrigerator mothers.” Clinicians have urged scientists to note these symptoms in a way that does not create a new diagnostic category and noting certain social, personality and language characteristics in family members has been crucial for nailing down the underlying biology.
Importantly, significant scientific discoveries in autism were made possible by looking directly at the brains of people with autism. This type of research has been made possible through the Autism BrainNet. This summary highlights the important role of studying brain tissue from individuals with autism to better understand people with autism across the lifespan, including those with known causes and unknown causes of autism spectrum disorder (ASD). See the section below entitled “Using brain tissue to understand causes of ASD” for more details on findings from brain tissue research in 2016.
Siblings show features of autism, but not to worry
This year, four studies assessed the broader autism phenotype in siblings, and other studies went further to look at psychiatric symptoms in siblings who were not diagnosed with autism. In the past, researchers mistakenly believed that siblings showed no symptoms of autism. In fact, adolescent, school age, and adult siblings of those with autism show elevated autism symptoms [1, 2] as well as categorical features of autism similar to those seen with autism [3], compared to those with no family history. High-risk infant sibling studies have shown that siblings of toddlers with autism, while not diagnosed with autism, have a higher rate of autism spectrum disorder (ASD) symptoms [4].

This is also consistent with the broader autism phenotype, with the last study indicating that sibling symptoms are observed across the lifespan. Unfortunately, signs of the broader autism phenotype puts siblings at risk for internalizing and externalizing behaviors like depression, psychological problems, and other behavioral issues [5, 6]. In addition to anxiety and depression, research this year showed increased risk of psychiatric comorbidities including ADHD and substance abuse disorders [7-9]. On the other hand, siblings who were within the typical range of Social Responsiveness Scale (SRS) scores didn’t show elevated sensory issues [10]. The goal of studying siblings of those with autism, again, is not to look for features which pathologize them, but to help identify features, challenges, and strengths that help them. Research published previously identifies the unique nature of sibling relationships, in that siblings of a person with autism view their relationship positively across the lifespan, whereas siblings of typically developing individuals tend to report positive feelings at a decreased rate in adulthood [11].
Understanding the causes of autism by studying sex differences

In addition to understanding siblings to help develop specialized services and supports, learning about siblings can help researchers understand the causes of autism and, specifically, why females are less likely to be diagnosed compared to males. New prevalence data from the CDC showed that the prevalence of autism is again at 1:68, perhaps showing a plateau in the rates of autism in the US. However, the difference in the rates between males and females still hovers around 4:1 depending on IQ [12]. Researchers this year showed that females may be able to hide symptoms because of better social abilities [13] and because they may be protected in some way from certain symptoms [14]. For example, those studying infants at risk for autism show that baby girls with autism show increased attention to social stimuli compared to baby boys [15]. This difference may affect how they express symptoms later on. Finally, preliminary studies this year suggest a slight bias in diagnostic instruments [16] and evidence of camouflaging autism symptoms in females [17].

There are likely multiple reasons behind the male sex bias in autism, but few have received any empirical study. This year, the Autism Sisters Project began recruiting at the Icahn School of Medicine at Mount Sinai. This study is poised to understand why females are not diagnosed as often, including differences in IQ and underlying genetic factors. Of importance, the study is focusing on the undiagnosed sister of individuals with ASD. As much as studying siblings with autism may help researchers understand sex differences in autism, so will actually studying males and females with ASD. Donna Werling from USCF looked at genes expressed in the brains of males and females with and without autism to understand sex differences in gene expression, particularly in those genes associated with autism. She found that it was not ASD risk genes that show differences, but those that are involved in neural pathways associated with autism, like microglia and the immune system, that show sex differences. The male bias in this gene expression may be what modulates ASD risk [18]. A male sex bias is not unusual across neurodevelopmental disorders, and so understanding its role in autism diagnoses may be informative of disorders like ADHD and anxiety as well. Just like there are fewer females diagnosed with autism, there are fewer brains of females to study, slowing scientists’ understanding of ASD. In order to learn more about how women with ASD can participate, click here.
More to learn about genetics associated with ASD

Even more new risk genes were discovered this year, and/or replicated in different cohorts. These investigations went beyond “autism” vs. “no autism” to specific features of autism, with the goal of understanding what genes lead to what behavioral features of ASD. For example, several studies found associations between a gene called POGZ and autism, particularly autism with intellectual disability [19-23]. POGZ is a gene that makes a protein that affects the expression of other genes. Therefore, the mutation of this gene produces disruption in the expression of several genes, rather than just one. Similar specific behavioral features are found with mutations of TRIP12 or DYRK1A, which also leads to widespread, rather than specific, changes in gene expression with a particular form of autism: autism with intellectual disability [24, 25]. By further investigating individuals for whom substantial amounts of data is available, including cognitive ability and comorbid medical conditions, the causes of these features will be better understood and will hopefully lead to better treatments. Researchers have also better identified how genes seen in other disorders but cause autism are transmitted, for example via maternal [26, 27] or paternal [28] pathways, influenced by things like paternal age [29], which might aid genetic counseling. Contrary to this idea, however, is the finding that individuals with either a known genetic cause of autism or autism where there is no known cause (i.e., idiopathic) have a considerable amount of overlap in mutations in the brain which affect how genes are turned on and off – in other words “epimutations” [29, 30]. This is further evidence that beyond the way DNA is sequenced, factors that affect how genes are activated are important to autism etiology as well. Epigenetic markers are known to be sensitive to environmental exposures of different types and insight into these pathways continue to open the door to understanding gene/environment interactions in autism.

And it’s not just about pure genetics, it’s about interactions between genes and the environment.
Genetics plays a huge role in the causes of autism, but this year researchers dove even deeper into the multifactorial causes of autism, specifically the role of genetics and the environment. The environment includes, broadly speaking, anything from toxic chemicals to age of the parent. It includes sociological, pharmacological, toxicological, and medical exposures.
This year saw two epidemiological studies examining the interaction between genes and the environment, but this time the investigation expanded to include who carried the genetic mutation and how autism was defined. First, studying the genotype of mothers showed that a particular mutation of the serotonin receptor gene and a high level of stressors during pregnancy produced a higher risk for having a child with autism than those without this same mutation [31]. Rather than using these factors to understand autism risk, others are going beyond to understand symptoms within autism. For example, using the Simons Simplex Collection, scientists showed that boys with autism who had genetic markers of mutations called copy number variations, together with exposure to an environmental exposure, showed the most severe autism symptoms, marked by repetitive behaviors and cognitive challenges [32]. The study is the first to look at type and severity of symptoms following multiple risk factors rather than a diagnosis, and the idea of understanding multiple risk factors for symptoms, rather than diagnosis itself, needs further study. Animal models of autism found that paternal age, a commonly accepted risk factor for autism spectrum disorder, combined with a mutation of a gene that affects synaptic development, results in certain symptoms of ASD in this model [33]. More fine-grained analysis of autism symptoms, rather than an autism diagnosis per-se, is needed to better understand the causes of autism. It’s also important to understand environmental factors because in some cases, like those of chemical and toxicological exposures, these can be controlled through regulatory means. Many studies have linked air pollution to autism [34] and in early July a landmark consensus statement authored by over 30 scientists, physicians, and public health experts was published which calls for the reduction of toxic chemical exposures to possibly reduce the risk of many developmental disorders [35]. So far, the only established way to protect against autism has been dietary folic acid supplementation [36], so reduction of modifiable risk factors should be a focus of future public health research.
Another potentially modifiable risk factor is maternal infection during pregnancy. Of course, not all cases of maternal infection are preventable, but some of them are. This year, a study revealed that neither having the flu, nor being vaccinated against the flu during pregnancy, was shown to contribute to autism risk in children [37]. However, maternal immune response during pregnancy was linked to a specific behavioral phenotype of autism, specifically those with intellectual disabilities [38]. According to animal models, the effects of altering the immune system function early in cell formation may lead to longer lasting elevations in chemokines (which are immune chemicals associated with autism) than previously thought [39]. This may be attributed to long lasting changes in gene expression patterns, regulated via epigenetic mechanisms [40, 41], resulting in an increase of methylation of genes and producing effects across generations. These findings converge with other research that demonstrates similar methylation patterns in individuals with ASD, even without immune system challenges during early life.
For years, some autism researchers have observed the presence of antibodies to brain tissue in some mothers of children with autism. This year, researchers looking at animal models discovered that they may be acting through an autism risk gene [42]. Also, the increased risk may be particularly elevated in mothers with specific medical conditions [43]. While scientists remain cautious about translating these findings to a commercialized method of determining autism risk, they continue to provide insights into the neurobiology of autism, and especially the immune system.
Using brain tissue to understand causes of ASD

Brain tissue research will also help researchers better identify causes of different types of autism so that better treatments can be developed. For example, one of the more challenging and debilitating medical comorbidities associated with autism is seizures and epilepsy. A study of brains of individuals with both autism and autism and epilepsy show increased numbers of glial cells. These cells are not neurons, rather they provide support and protection to brain cells [44]. The glial cell numbers were highest in those with restricted and repetitive behaviors, but, interestingly enough, the number of glia go down over time in individuals with autism, but up in those without autism. This suggests that the glial cells contribute to autism severity and cause. Similar comparisons to other disorders associated with autism were made studying amyloid B precursor protein and their metabolites. These molecules are associated with Alzheimer’s disease but also have a host of other functions that are not pathogenic. For example, they can affect neuroinflammation and normal cellular activity. In autism, levels of these proteins were reduced in brain and plasma, but elevated in individuals with Fragile X syndrome [45]. This suggests that these amyloid B proteins are involved with both disorders, and may be a target of interventions in the future.
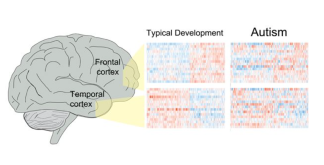
Figure found in Parikshak et al., 2016
Brain tissue research goes beyond identifying treatment targets to helping researchers understand how the brains of people with autism work on a cellular level. This year, two studies demonstrated that in addition to mutations in autism risk genes, mutations in areas of the gene that control the function of autism risk genes are also affected [30, 46]. What is also interesting is that regardless of the symptoms or causes of autism, the pattern of gene activity was similar in those with autism, validating a much smaller study from years ago [46]. These results also reiterate the importance of early intervention for treatment of debilitating autism symptoms, since both genes identified recently that control brain development peak during the first few years of life. It is important for all families, regardless of whether or not they are directly affected by autism, to learn more about brain tissue donation. You can register for more information by clicking here.
Should clinicians think in terms of autism diagnosis, or in terms of symptoms?

This year showed the shared features between autism and many other disorders like Phelan-McDermid syndrome, mutations of chromosome 16, Dup15, and even schizophrenia. In particular, disorders don’t just share autism symptoms; they show similar neurological and cognitive features as well [27, 47]. So how much is specific to autism, and how much is related to behavioral, neurological, and other medical issues that are seen without an autism diagnosis? And do these genetic findings explain certain symptoms associated with autism, but not core to autism? It has been argued that classifying individuals based on specific symptom dimensions, such as the presence of abnormal behaviors, absence of other behaviors, and cognitive ability may help clinicians better distinguish cross disorders [48, 49]. This idea is not new, with a recent movement towards a new way of thinking towards autism diagnosis [50]. New findings from the brains of individuals with a diagnosis of autism or schizophrenia show significant overlap between the gene transcription in the brains of people with either autism or schizophrenia, but not bipolar disorder [51]. The authors conclude that these two disorders share many genes associated with synapse development, and the formation of connections across different brain regions. Therefore, these disorders may not be totally different at the biological level. Rather than thinking of autism as a whole, early signs of autism can also be linked to specific genetic markers, which may explain autism symptoms, but not autism as a diagnosis. This includes mutations of the oxytocin receptor on later empathy [52] and dopamine receptors on a core feature – initiating joint attention [53]. This idea has enormous implications for autism research and treatment, as it implies a switch in the way autism is identified. It has been suggested that behavioral symptoms, combined with biological and environmental variables, should be combined to lead to categories, rather than diagnosis of disorders. This is called Research Domain Criteria, or RDOC.
Autism can also be very difficult to diagnose, but this year two new studies suggested that the process can be streamlined, at least a little bit. In school age verbal children, a new instrument called the Autism Symptom Inventory (ASI) was good at diagnosing autism in about 20 minutes [54]. Another instrument, which doesn’t have a name yet, combines three short instruments (including the ASI) and was also promising, especially in terms of studies aiming to understand the causes of autism, both genetic and environmental [55]. These studies offer hope to large scale epidemiological studies seeking to identify and characterize individuals with autism, although right now their ability to identify different subtypes which may be amenable to specialized treatments is limited.
When it comes to intervention, earlier is best, but not the only option

The most remarkable evidence of the effectiveness of early intervention has come from longitudinal studies – those that study an intervention YEARS after it was delivered. If early intervention improves brain connectivity and allows for connections to be formed to alleviate autism symptoms, the effects may not be seen right away – it may take years. They can take the form of an intervention study that follows families for a long time, or by investigating factors early on that predicted improvement at school age and beyond. This year saw both. In 2010, a gold standard randomized clinical trial study out of the UK looked at a parent-delivered intervention focusing on communication, and, while they found it showed promise, it didn’t produce improvements in symptom severity [56]. The initial findings were hopeful, but also disappointing. However, when they followed up on these children five years later, the training of the parents to deliver the intervention resulted in a reduction of autism symptoms [57]. The findings are important for many reasons. First, autism intervention is a journey, not necessarily a destination, and interventions delivered early on may alter the trajectory of symptoms [57]. Second, parents can deliver interventions in a wide variety of settings in a way that is more intensive than limited clinic time, and an intervention targeted at one set of autism symptoms like social communication may also affect others like repetitive behaviors [58]. This does not mean that trained Applied Behavioral Analysis (ABA) therapists and intervention delivered by trained professionals should be abandoned. Parent-delivered interventions are a supplement at ages when kids spend most of their time with parents rather than schools. Another important thing to remember about early intervention is that more data published this year shows that for a percentage of children, a diagnosis is not possible at two years of age. A group of children who show some symptoms but don’t meet criteria at two years of age do end up with a diagnosis by three years of age [59] despite being seen by well-trained, very experienced clinicians. Early intervention may help those who don’t have an actual autism diagnosis yet. Studying infants with autism has also been instrumental in determining not only interventions, but the nature of autism itself. For years, people assumed that the reduced eye contact in people with autism was because they were actively averting the eyes, found eye contact aversive, and didn’t want to look at the gaze of the other person. However, this isn’t the case. At least early in life, infants with autism don’t actively avert gaze, they just aren’t that interested in looking at the eyes and don’t get the same social signals from eye contact as those with autism do [60].
Parents as methods of treatment delivery
Parent-delivered interventions can be used at different times, again to supplement, rather than replace, other treatments delivered in clinical settings. Parent training, not the less intensive parent education, on behavior management techniques improved adaptive behavior and daily living in children with autism. However, these gains were mostly seen in those with average intellectual functioning [61]. This suggests that not all individuals respond to parent-delivered interventions. And it isn’t just used in isolation. It enhances the efficacy of drugs to alleviate ADHD in those with autism [62]. Parent training may seem like an easy solution, but in the real world setting of parents and trainers, it is very complicated [63].
What can predict who will respond to what treatment?
There have also been advances in pharmacological treatments of autism, but they always struggle with improving behavior or outcome, not specific to core autism symptoms. Oxytocin, a naturally occurring hormone, has shown mixed results in improving different aspects of autism-related behavior, including face recognition, social behavior, and empathy [64]. Looking at the effect of oxytocin on the brain, it improves connectivity between areas of the brain involved in reward and those involved in perception of social communication cues in children with autism [65]. However, it isn’t simple, and, as it turns out, that makes the story more promising. People with mutations of the oxytocin receptor have different types of mutations. These different types of mutations in people with autism lead to different patterns of this connectivity [66] as well as the ability to recognize faces [67]. Finally, these different mutations also predict the behavioral response to oxytocin – in other words, whether or not this hormone produces improvements in social abilities [68]. These different studies are a perfect illustration of how personalized medicine will improve autism treatment. Those with particular types of genetic differences will respond better to oxytocin treatment than others, which will speed up people receiving the right type of intervention.

In addition to genetic markers predicting treatment response, advances in other biomarkers to predict treatment response have been made as well. Individuals who were more responsive to Pivotal Response Treatment (PRT) showed a specific pattern of pre-treatment brain activity when presented with a social situation on a video [69]. In fact, it predicted response to treatment better than any baseline behavioral measures. In the future, just like looking at the genetic makeup of people with autism, understanding their underlying brain function before treatment can help get the people into the treatments that would benefit them the most.
The whole purpose of improvements in autism diagnosis and interventions is to deliver services to individuals that need them. So, how are insurance mandates doing in terms of identifying individuals with autism and providing them with the treatments they need? This year, David Mandell at the University of Pennsylvania demonstrated with data obtained through insurance companies that these mandates are increasing the number of people receiving services. That’s the good news. The bad news is that the increase is not nearly as much as it should be keeping in pace with the prevalence of autism. So, he concludes, these mandates are necessary but not sufficient to provide services to all that need them [70]. In addition, there are acknowledged gaps in what pediatricians know about non-medical treatments and services in their areas, and what parents need them to understand [71].
In summary, this year saw research that helps understand the causes of autism; includes siblings to provide better services to the entire family; showed promise of the concept of “personalized medicine” everyone has heard so much about; demonstrated the long term, not just short term effects of behavioral interventions and the importance of parents and caregivers; and emphasized the need to better understand features of individuals with autism rather than just the straight diagnosis of ASD.
References:
- Ruzich, E., et al., The Autism-Spectrum Quotient in siblings of people with Autism. Autism Res, 2016. 9(10): p. 1114.
- Ruzich, E., et al., Subgrouping siblings of people with autism: Identifying the broader autism phenotype. Autism Res, 2016. 9(6): p. 658-65.
- Tsang, T., K. Gillespie-Lynch, and T. Hutman, Theory of Mind Indexes the Broader Autism Phenotype in Siblings of Children with Autism at School Age. Autism Res Treat, 2016. 2016: p. 6309189.
- Charman, T., et al., Non-ASD outcomes at 36 months in siblings at familial risk for autism spectrum disorder (ASD): A baby siblings research consortium (BSRC) study. Autism Res, 2016.
- Walton, K.M., Risk Factors for Behavioral and Emotional Difficulties in Siblings of Children With Autism Spectrum Disorder.Am J Intellect Dev Disabil, 2016. 121(6): p. 533-549.
- Fullerton, J.M., et al., Siblings of children with life-limiting conditions: psychological adjustment and sibling relationships.Child Care Health Dev, 2016.
- Jokiranta-Olkoniemi, E., et al., Risk of Psychiatric and Neurodevelopmental Disorders Among Siblings of Probands With Autism Spectrum Disorders. JAMA Psychiatry, 2016. 73(6): p. 622-9.
- Butwicka, A., et al., Increased Risk for Substance Use-Related Problems in Autism Spectrum Disorders: A Population-Based Cohort Study. J Autism Dev Disord, 2016.
- Miller, M., et al., School-age outcomes of infants at risk for autism spectrum disorder. Autism Res, 2016. 9(6): p. 632-42.
- Hilton, C.L., et al., Sensory Responsiveness in Siblings of Children with Autism Spectrum Disorders. J Autism Dev Disord, 2016.
- Seltzer, M.M., G.I. Orsmond, and A.J. Esbensen, Siblings of individuals with an autism spectrum disorder: Sibling relationships and wellbeing in adolescence and adulthood. Autism : the international journal of research and practice, 2009. 13(1): p. 59-80.
- Christensen, D.L., et al., Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years–Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ, 2016. 65(3): p. 1-23.
- Rynkiewicz, A., et al., An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol Autism, 2016. 7: p. 10.
- Constantino, J.N., Data from the Baby Siblings Research Consortium confirm and specify the nature of the female protective effect in autism: A commentary on Messinger et al. Mol Autism, 2016. 7: p. 32.
- Chawarska, K., et al., Enhanced Social Attention in Female Infant Siblings at Risk for Autism. J Am Acad Child Adolesc Psychiatry, 2016. 55(3): p. 188-95 e1.
- Beggiato, A., et al., Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res, 2016.
- Lai, M.C., et al., Quantifying and exploring camouflaging in men and women with autism. Autism, 2016.
- Werling, D.M., N.N. Parikshak, and D.H. Geschwind, Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun, 2016. 7: p. 10717.
- Wang, T., et al., De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat Commun, 2016. 7: p. 13316.
- Stessman, H.A., et al., Disruption of POGZ Is Associated with Intellectual Disability and Autism Spectrum Disorders. Am J Hum Genet, 2016. 98(3): p. 541-52.
- Loviglio, M.N., et al., Identification of a RAI1-associated disease network through integration of exome sequencing, transcriptomics, and 3D genomics. Genome Med, 2016. 8(1): p. 105.
- Tan, B., et al., A novel de novo POGZ mutation in a patient with intellectual disability. J Hum Genet, 2016. 61(4): p. 357-9.
- Hashimoto, R., et al., Whole-exome sequencing and neurite outgrowth analysis in autism spectrum disorder. J Hum Genet, 2016. 61(3): p. 199-206.
- Bramswig, N.C., et al., Identification of new TRIP12 variants and detailed clinical evaluation of individuals with non-syndromic intellectual disability with or without autism. Hum Genet, 2016.
- van Bon, B.W., et al., Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry, 2016. 21(1): p. 126-32.
- Connolly, S., et al., A genome-wide investigation into parent-of-origin effects in autism spectrum disorder identifies previously associated genes including SHANK3. Eur J Hum Genet, 2016.
- Duyzend, M.H., et al., Maternal Modifiers and Parent-of-Origin Bias of the Autism-Associated 16p11.2 CNV. Am J Hum Genet, 2016. 98(1): p. 45-57.
- Isles, A.R., et al., Parental Origin of Interstitial Duplications at 15q11.2-q13.3 in Schizophrenia and Neurodevelopmental Disorders. PLoS Genet, 2016. 12(5): p. e1005993.
- Yuen, R.K., et al., Genome-wide characteristics of de novo mutations in autism. NPJ Genom Med, 2016. 1: p. 160271-1602710.
- Sun, W., et al., Histone Acetylome-wide Association Study of Autism Spectrum Disorder. Cell, 2016. 167(5): p. 1385-1397 e11.
- Hecht, P.M., et al., Maternal serotonin transporter genotype affects risk for ASD with exposure to prenatal stress. Autism Res, 2016. 9(11): p. 1151-1160.
- Jane Webb, S., et al., Severity of ASD symptoms and their correlation with the presence of copy number variations and exposure to first trimester ultrasound. Autism Research, 2016: p. n/a-n/a.
- Yoshizaki, K., et al., Paternal Aging Affects Behavior in Pax6 Mutant Mice: A Gene/Environment Interaction in Understanding Neurodevelopmental Disorders. PLoS One, 2016. 11(11): p. e0166665.
- Lam, J., et al., A Systematic Review and Meta-Analysis of Multiple Airborne Pollutants and Autism Spectrum Disorder. PLoS One, 2016. 11(9): p. e0161851.
- Bennett, D., et al., Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement.Environ Health Perspect, 2016. 124(7): p. A118-22.
- Gao, Y., et al., New Perspective on Impact of Folic Acid Supplementation during Pregnancy on Neurodevelopment/Autism in the Offspring Children – A Systematic Review. PLoS One, 2016. 11(11): p. e0165626.
- Zerbo, O., et al., Association Between Influenza Infection and Vaccination During Pregnancy and Risk of Autism Spectrum Disorder. JAMA Pediatr, 2016.
- Jones, K.L., et al., Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry, 2016.
- Rose, D.R., et al., Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav Immun, 2016.
- Weber-Stadlbauer, U., et al., Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol Psychiatry, 2016.
- Richetto, J., et al., Genome-wide DNA Methylation Changes in a Mouse Model of Infection-Mediated Neurodevelopmental Disorders. Biol Psychiatry, 2016.
- Brimberg, L., et al., Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice.Mol Psychiatry, 2016. 21(12): p. 1663-1671.
- Krakowiak, P., et al., Autism-specific maternal anti-fetal brain autoantibodies are associated with metabolic conditions. Autism Res, 2016.
- Menassa, D.A., C. Sloan, and S.A. Chance, Primary olfactory cortex in autism and epilepsy: increased glial cells in autism. Brain Pathol, 2016.
- Ray, B., et al., Finding novel distinctions between the sAPPalpha-mediated anabolic biochemical pathways in Autism Spectrum Disorder and Fragile X Syndrome plasma and brain tissue. Sci Rep, 2016. 6: p. 26052.
- Parikshak, N.N., et al., Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature, 2016.
- Steinman, K.J., et al., 16p11.2 deletion and duplication: Characterizing neurologic phenotypes in a large clinically ascertained cohort. Am J Med Genet A, 2016. 170(11): p. 2943-2955.
- Foss-Feig, J.H., et al., Re-conceptualizing ASD Within a Dimensional Framework: Positive, Negative, and Cognitive Feature Clusters. J Autism Dev Disord, 2016. 46(1): p. 342-51.
- Schwarz, E., H. Tost, and A. Meyer-Lindenberg, Working memory genetics in schizophrenia and related disorders: An RDoC perspective. Am J Med Genet B Neuropsychiatr Genet, 2016. 171B(1): p. 121-31.
- London, E.B., Categorical diagnosis: a fatal flaw for autism research? Trends Neurosci, 2014. 37(12): p. 683-6.
- Ellis, S.E., et al., Transcriptome analysis of cortical tissue reveals shared sets of downregulated genes in autism and schizophrenia.Transl Psychiatry, 2016. 6: p. e817.
- McDonald, N.M., J.K. Baker, and D.S. Messinger, Oxytocin and parent-child interaction in the development of empathy among children at risk for autism. Dev Psychol, 2016. 52(5): p. 735-45.
- Gangi, D.N., et al., Dopaminergic variants in siblings at high risk for autism: Associations with initiating joint attention. Autism Res, 2016. 9(11): p. 1142-1150.
- Bishop, S.L., et al., The autism symptom interview, school-age: A brief telephone interview to identify autism spectrum disorders in 5-to-12-year-old children. Autism Res, 2016.
- Newschaffer, C.J., et al., Development and validation of a streamlined autism case confirmation approach for use in epidemiologic risk factor research in prospective cohorts. Autism Res, 2016.
- Green, J., et al., Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet, 2010. 375(9732): p. 2152-60.
- Pickles, A., et al., Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet, 2016.
- Harrop, C., et al., The impact of caregiver-mediated JASPER on child restricted and repetitive behaviors and caregiver responses.Autism Res, 2016.
- Zwaigenbaum, L., et al., Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Res, 2016. 9(7): p. 790-800.
- Moriuchi, J.M., A. Klin, and W. Jones, Mechanisms of Diminished Attention to Eyes in Autism. Am J Psychiatry, 2016: p. appiajp201615091222.
- Scahill, L., et al., Effect of Parent Training on Adaptive Behavior in Children With Autism Spectrum Disorder and Disruptive Behavior: Results of a Randomized Trial. J Am Acad Child Adolesc Psychiatry, 2016. 55(7): p. 602-609 e3.
- Smith, T., et al., Atomoxetine and Parent Training for Children With Autism and Attention-Deficit/Hyperactivity Disorder: A 24-Week Extension Study. J Am Acad Child Adolesc Psychiatry, 2016. 55(10): p. 868-876 e2.
- McKnight, L.M., M.P. O’Malley-Keighran, and C. Carroll, ‘Just wait then and see what he does’: a speech act analysis of healthcare professionals’ interaction coaching with parents of children with autism spectrum disorders. Int J Lang Commun Disord, 2016. 51(6): p. 757-768.
- Ooi, Y.P., et al., Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry, 2016.
- Gordon, I., et al., Intranasal Oxytocin Enhances Connectivity in the Neural Circuitry Supporting Social Motivation and Social Perception in Children with Autism. Sci Rep, 2016. 6: p. 35054.
- Hernandez, L.M., et al., Additive effects of oxytocin receptor gene polymorphisms on reward circuitry in youth with autism. Mol Psychiatry, 2016.
- Westberg, L., et al., Variation in the Oxytocin Receptor Gene Is Associated with Face Recognition and its Neural Correlates. Front Behav Neurosci, 2016. 10: p. 178.
- Watanabe, T., et al., Oxytocin receptor gene variations predict neural and behavioral response to oxytocin in autism. Soc Cogn Affect Neurosci, 2016.
- Yang, D., et al., Brain responses to biological motion predict treatment outcome in young children with autism. Transl Psychiatry, 2016. 6(11): p. e948.
- Mandell, D.S., et al., Effects of Autism Spectrum Disorder Insurance Mandates on the Treated Prevalence of Autism Spectrum Disorder. JAMA Pediatr, 2016. 170(9): p. 887-93.
- Levy, S.E., et al., Shared Decision Making and Treatment Decisions for Young Children With Autism Spectrum Disorder.Acad Pediatr, 2016. 16(6): p. 571-8.

